Bacteria that turns to stone
Bacteria that turns to stone
“Daadii (दादी), please tell me a story”, I pleaded with my grandmother for the millionth time and, as always, she obliged with a smile. That night, my daadii recounted the story of Ahilya who, cursed by her husband for infidelity, was turned to stone but was eventually freed by Lord Rama. “Daadi, living beings cannot turn to stone”, I said with the assurance of an all-knowing young scholar with biology lessons to his credit. I thanked her for the story and went to sleep.
Imagine my surprise, when years later, I found myself staring through a microscope to investigate a bacterium called Sporosarcina pasteurii (SP) and watching it slowly turn to ‘stone’! This was not a case of my eyes playing tricks on me; rather I was witnessing the process of biomineralization. Biomineralization refers to the process of mineral precipitation due to chemical alteration of the environment induced by the life processes. Many organisms are capable of this phenomenon, and even diminutive bacteria are able to achieve this. For unicellular organisms such as bacteria, the biomineralization process can be either extracellular or intracellular. Biomineralization is possibly one of the largest ‘manufacturing’ processes undertaken by Earth and is closely associated with the advent of life on this planet. Many opine that biomineralization processes became widespread toward the end of the pre-Cambrian period, possibly around 3.8 billion years ago [1]. Biomineralization also led to the substantial cooling of the planet as the process can sequester molecules like carbon dioxide by converting them into carbonates. A seashell is a product of biomineralization. The next time you walk along a seashore, take a few moments to find one and imagine how that tiny bit of ‘rock’ played a part in determining the history of our beloved Prithvi (Sanskrit term for Earth).
Many organisms have been implicated for their role in biomineralization. In the context of bacteria, it is known that certain species are able to participate in this process, although several aspects of the biophysics of the process are still being investigated. Our bacterium, SP, has been in the limelight for its ability to precipitate calcium carbonate under the right conditions. Certain features of biomineralization process are known – for example, what kind of ‘food’ the bacterium prefers in such situations, the effect of media pH, etc. The following sequence of reactions has typically been suggested to lead to the biomineralization process for SP [2]:
CO(NH2)2 + H2O ureolysis -> NH2COOH + NH3 (1)
NH2COOH + H2O -> NH3 + H2CO3 (2)
2NH3 + 2H2O -> 2NH4+ + 2OH- (3)
2OH- + H2CO3 -> CO32- + 2H2O (4)
Cell + Ca+2 -> Cell-Ca+2 (5)
Cell-Ca+2 + CO32- -> Cell-CaCO3 (6)
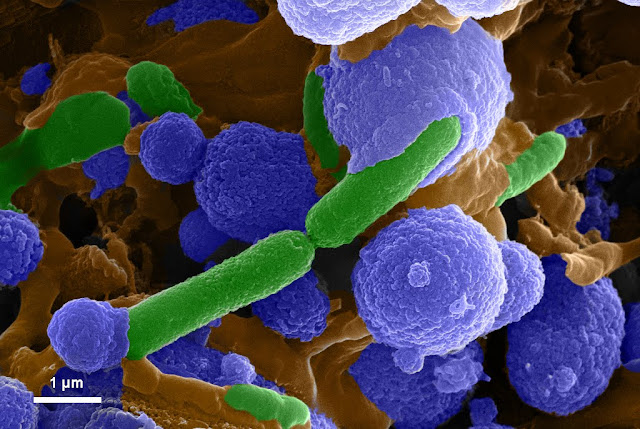
Equation (6) remains controversial. Many researchers suggest that the bacterial cell walls themselves serve as nucleation sites since bacterial cell surfaces carry negatively charged groups and thus facilitate the binding of cations (e.g. Ca2+) on the cell surface. However, this idea has been contested by other researchers who assert that certain cell protection mechanisms prevent mineral encrustation in the exact vicinity of the bacteria. They theorize that mineral precipitation occurs at a distance from the cell walls and not directly on the cell wall. You might think that this disagreement could be easily resolved by simply observing the process. However, the dynamics of the process are such that direct observation, while the crystals are nucleating, is challenging. In our work, we settled the question by using a specially prepared gel suspension to observe the cells during this process. Quite interestingly, we were able to observe the slow calcification of the cell surface, wherein the nucleation initiates as nano-crystals. The two images below represent samples of our results. The scanning electron microscope images has been false colored such that the bacteria are green, the calcium carbonate crystals are blue and biological glue is golden.
Image Credits: Dr. Tanushree Ghosh, University of Alberta
Our findings were recently published in the journal PLOS ONE.
Research into the biophysical aspects of biomineralization has caught the attention of many researchers due to potential applications in creating green structures and self-healing buildings. For example, one wishes to restore a thousand year old murti, which has developed internal cracks. Maintenance of such a structure poses severe challenges to conservationists: unsuitable repairs can significantly degrade the quality of a heritage structure and possibly devalue it by an unacceptable margin. Here one could employ biomineralization bacteria to act as micro-masons, to go in and heal the cracks by turning into stone. The same concept can be utilized to make ‘green’ bricks. There are startups today that are actually translating these ideas into reality and carving out a biomineralization market niche for themselves.
References:
1. Skinner, H.C.W. and Jahren, A.H., 2003. Biomineralization. Treatise on geochemistry, 8, p.682.
2. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0210339
2. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0210339
About the author: Dr. Aloke Kumar is currently an Assistant Professor at Indian Institute of Science, Bangalore. He tweets at @aalokelab


A nice story with great scientific value, I know the author personally, he is a fantastic and motivated researcher, I have had the opportunity to visit his biomineralization lab at IISc Bangalore, India, Department of Mechanical Engineering. They are doing great job for humanity.. I wish him and his team all the very best for their future work..
ReplyDelete